Biofuels for a Cleaner Future
Biofuels by themselves are not going to stop global warming. And they can only affect air pollution locally. But biofuels can make for a cleaner, brighter future: cleaner dishes and brighter clothes, that is. And we might be able to ditch some dangerously exotic household cleaning chemicals while we are at it.
The biofuels won’t directly go into detergents, however. Instead, the brewing of biofuels could allow us to once again use one of the safest, most effective, household cleaner ingredients.
A Forgotten Natural Household Cleaner
Soap is an ancient cleaning product. Mix fat and ashes together and you get soap. Fat contains fatty acids. Wood ashes contain alkalies based on sodium and potassium ions. These ions combine with the fatty acids to produce molecules which are fat soluble on one end and water soluble on the other. Soapy water can thus wash grease from clothes, dishes, etc.
The power of such simple soaps made with natural ingredients is limited, however. There are other alkaline minerals which can combine with fatty acids: calcium, magnesium, iron, etc. When these ions replace the more alkaline sodium and potassium, the result is not water soluble. The result is soap scum.
Dirt contains such soap scum forming metals. To wash clothing with just soap is to form soap scum.
Hard water has such ions even before you add dirt. With hard water you can get soap scum even when washing dishes.
What you need is a negative ion which bonds tightly to calcium, magnesium, iron, etc. so such ions don’t bond to the fatty acids. But you also need a negative ion which doesn’t attack undissolved metals so vigorously that you destroy your dishwasher, your washing machine, or your plumbing. The latter consideration rules out chloride and sulfate ions.
Phosphate ions are Just Right. They bond tightly to calcium; your bones and teeth are made of calcium phosphate. Phosphoric acid is the main ingredient in Naval Jelly rust remover. (Try using Coca Cola, which also contains phosphoric acid, on rusty objects.)
For years, sodium tripolyphosphate was used with soap to make detergents. The soap cut grease and the phosphates kept other metallic minerals from turning soap into soap scum.
Phosphate ions are safe. Phosphates are an essential nutrient for all life.
But there is a problem with using an essential nutrient for life as a cleaning ingredient: you get more life, more life than you want. Phosphate detergents were causing massive algae blooms in rivers and lakes, forming slimy green scums, scums which sucked the oxygen out of the water when they rotted, killing the fish.
So, phosphates were banned from household laundry detergents decades ago. (They still use phosphates for commercial laundry detergents. That’s how hotels and hospitals can get their towels and sheets clean.)
More recently, many states banned phosphates from even household dishwasher detergents, to the point where it’s hard to find phosphate containing dishwasher detergent even where phosphates are still legal.*
The result is disgusting scum buildup inside modern dishwashers. And if you don’t clean the scum often enough, you get scum on your dishes. Yuck!
We could put phosphates back in dishwasher detergent, and even in laundry detergent, if we were to embrace biofuels.
Biofuels to the Rescue!
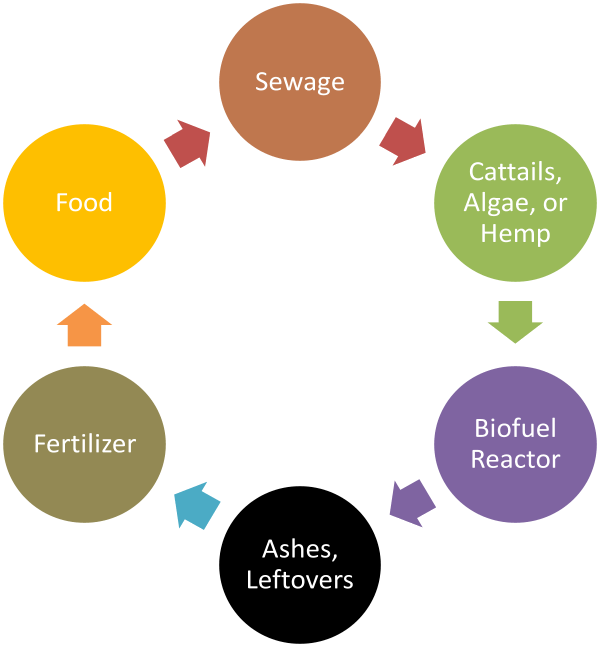
Nutrient rich water produces excess life. This excess life can be used for fuel. Grow cattails in nutrient rich treated wastewater and you get enormous amounts of starch. Break down this starch with already available enzymes and you get sugar suitable for fermentation into alcohol. (See Alcohol Can Be a Gas by David Blume.)
Or you could embrace the scum. Some varieties of algae make significant amounts of fatty acids, fatty acids that can be used to make biodiesel. Instead of fretting about green scum, run the treated wastewater through a scum farm to make fuel.

Would such arrangements be profitable? Probably not, or we would see more biofuel plants now. Maybe if we had a carbon tax such plants might be profitable. But even then the answer is probably “No.” The problem we have is that sewerage treatment plants are generally near towns and cities. There is no space handy for cattail or algae farms. You would need to pump the treated wastewater out to farmland where this is room and sunlight. The pumping would generally be uphill.
It might be worth having a government pay this cost – if the required subsidy is less than the costs we are already experiencing because we cannot use phosphates in our cleaning products. (Doing this cost-benefit calculation is beyond our resources. This is a job for think tanks and/or government bureaucrats.)
When doing such cost-benefit calculations, consider also the toxicity of cleaning agents required because we cannot use phosphates. Phosphates are simple and safe. Some of the chemicals added to our soaps are turning Americans into whiny gurly-men and worse. (Whether these estrogen mimics are added to replace phosphates or for other reasons is on our To Be Determined list. Maybe some longtime environmental activists in the audience already know the answer. If so, please contact us!)
The Looming Age of Peak Phosphorus
There is yet another reason for recovering phosphates from wastewater vs. simply banning phosphate detergents: human waste also contains phosphates. According to this Mother Earth News article from 1970, phosphate detergents contributed 1.5 to 2 pounds of phosphorus per person per year into our lakes and streams. Human waste contributed 1.4 pounds of phosphorus per person per year. In other words, we are still adding algae to our lakes and rivers even after banning phosphate detergents.
Now here is the scary bit: all life uses phosphates, but phosphorus is not an abundant chemical! Life persists because nature recycles phosphorus. When we flush our waste into rivers, we are short circuiting this cycle. We have been getting away with this by mining phosphorus and using it for fertilizer.
But there aren’t many phosphorus deposits out there to mine. The main deposits are in Morocco. When those deposits run out, we are in trouble. Soon, we will need to recycle. The only question is: Will we will recycle the smart way or some stupid way?
For stupid solutions to future environmental problems, read the eco-dystopias Earth and Existence by liberal science fiction writer David Brin. In one of these novels he portrays a future where men piss into special containers for special pickup in order to recover scarce phosphorus for fertilizer. (The Romans did something similar in order to make ammonia-based cleaners. Bleah!)
Compared to such an inconvenient and messy solution to Peak Phosphorus, spending some government money to pipe treated wastewater to cattail and algae farms is dirt cheap. Once the starch is fermented from the cattails and/or the biodiesel extracted from the algae, the remaining biomass can be burnt and the ashes used for phosphate fertilizer.
As for which level of government should provide the subsidy, ask, “Who benefits?” Sewer plants benefit those downstream from cities more than the cities themselves. Therefore, a government at a higher level should pay some of the bill. Many rivers run through multiple states. Keeping them clean is thus an interstate matter, a matter actually appropriate for federal action(!)
*Check your state laws before using phosphate containing dishwasher detergent. Watersheds close to the ocean can tolerate more phosphates, since phosphates aren’t a big issue once water reaches the ocean. If your sewer system runs into a lake or a long river far upstream from the ocean, don’t use phosphate dish detergent even if legal in your state unless you know that your local sewage treatment plant takes extra steps to get the phosphates out.
You can comment on this chapter here.
(Link should open in a new window so you can see comments and text side by side.)